Date: 29 August, 2025
Location: Milan, Italy, and online
Attendee composition: APACS Vice-chair, APACS members, country liaisons, observers, donors, and APACS Secretariat (REACH Network, International Trachoma Initiative)
Meeting Brief:
The first meeting of the Advisory Panel on Azithromycin for Child Survival (APACS) was convened on Friday August 29, 2025. Established as a joint initiative of the REACH Network and the International Trachoma Initiative (ITI) and hosted in Milan, attendees joined both in person and virtually. APACS aims to provide independent expert advice on the safe, effective, and equitable allocation of donated azithromycin to reduce child mortality. The meeting included presentations from various stakeholders including donors (Gates Foundation, Pfizer) as well as an allocation request from Niger.
The three-hour meeting presented the APACS mandate, explored eligibility criteria for drug allocation and the need for standardized data collection and decision-making across participating countries in West Africa, particularly concerning mortality rates and antimicrobial resistance surveillance. Aligned with REACH Network values, the Panel emphasized country ownership and transparent processes while acknowledging the complexities of limited resources and varying data availability.
Meeting Agenda:
- the APACS mandate
- comments from donor partners
- discussion on allocation criteria, with an allocation discussion for Niger
- supply chain update.
Participants were encouraged to provide honest reflections during and after the meeting to strengthen the process.
Meeting Minutes:
Apologies having been received from the APACS Chair, the APACS Vice-Chair welcomed participants and chaired the meeting. A recorded message from the APACS Chair was played.
Donor Remarks
- Pfizer outlined their support of the REACH Programme since its beginning, including donation of antibiotics for child mortality studies. Given that the same azithromycin product is donated for both trachoma and REACH, coordinated oversight is vital, especially with fluctuating forecasts and capacity constraints, to ensure appropriate management of the supply chain and program direction.
- The Gates Foundation expressed excitement for APACS, seeing its potential to leverage experience from decades of trachoma work and rigorously apply science to inform decisions for donors and countries. They highlighted the importance of transparency for cross-country learning and assuring current and future donors about equity and effectiveness of application of scarce resources. They stressed that APACS should facilitate country ownership and cumulative learning.
APACs Mandate, Role, and Purview
The vice-chair described the APACS mandate as follows:
1. Rationale for APACS:
a. To allow for efficient and predictable azithromycin procurement and supply chain management as REACH grows.
b. To facilitate coordination amidst multiple azithromycin sources, investors, and partners.
c. To ensure transparency on criteria for starting and stopping mass treatment; report on monitoring, evaluation, and safety; and provide a clear data-driven approach for expanded partnerships.
2. Role of APACS: An independent advisory body on azithromycin stewardship for child survival that
a. Provides expert advice on technical and operational issues for safe, effective, equitable and rational use of azithromycin for this indication.
b. Serves as a technical resource for the REACH network and an independent advisory group to ITI, the REACH Network, and donors.
3. Purview of APACS:
a. Primarily focuses on recommendations for allocation and supply chain management of azithromycin.
b. Will also include understanding of safety data, interpreting global guidance on the use of azithromycin for child survival, generating additional guidance for application within the REACH Programme, and addressing other child survival program challenges.
c. The intent is not to duplicate existing work but to support activities related to azithromycin procurement, supply, and rational use within the REACH programme.
Discussion ensued, with the following key points raised:
- APACS primarily interprets guidelines, with development handled by country programs and WHO.
- Current WHO child survival guidelines (2020) are under revision and may not meet all country needs. WHO guidelines are recommendations, not strict rules.
- APACS can suggest solutions and help to build consensus where WHO guidelines are silent, out of date or unclear; countries are not obligated to follow WHO guidelines.
- Countries will be asked to submit quantification forms for drug forecasting early in the year to allow sufficient time for drug production.
- A cautionary remark was made about the potential difficulty in understanding and interpreting data if different countries adopt different standards, systems and processes for generating those data. The meeting urged standardization and international alignment to ensure comparability and long-term effectiveness.
Niger’s Program Presentation and Allocation Request
Program Overview: Niger’s REACH program, led by the Health Ministry, distributes azithromycin to children (1-59 months) door-to-door, aiming to reduce under-five mortality while monitoring for AMR.
Began in Dosso, Tahoua, and Maradi regions in June 2024 and expanded to Zinder and Agadez in 2025, with plans to include Diffa and Tillabéri later in 2025 (67 health districts, 328 centers, 3.28 million population).
Key distribution activities:
- First mortality surveys started in four regions (April 2024)
- First azithromycin distribution (Dosso, Tahoua, and Maradi regions, third quarter, 2024)
- Second distribution (February 2025)
- Third distribution (Agadez region added, June 2025).
Monitoring activities:
- Bi-annual mortality surveys (pregnancy history; baseline surveys began in April 2024 in four regions; the most recent mortality surveys in 2025 covered 198 health centers, 370,000 households, and 487,000 women
- Bi-annual AMR and pathogen surveillance in Maradi region
- Safety surveillance during distributions
- Monitoring of administrative scope, coverage, and protocol compliance.
The Niger REACH committee met in August 2025 to discuss and recommend criteria for starting and stopping MDA:
- Start: Under-five mortality ≥ 50 per 1,000 live births + AMR surveillance system in place.
- Stop: Under-five mortality < 25 per 1,000 live births, no effect after six distribution rounds or AMR (phenotypic tests on pneumococci) >30%.
- Evaluation surveys consider gender, child age, and caregiver age/education.
- Planned funding for targeted mortality surveys in 2026/2027.
- AMR data collected via nasal swabs from healthy, malaria, and pneumonia patients in participating health centers.
Lessons Learned: In a pilot integration project in one health district in Agadez, MDA with azithromycin was integrated with the child health day platform, which delivers vitamin A and albendazole to children under 5 years of age. The experience revealed several challenges, including increased burden on community health workers, decreased efficiency, and the need for further planning.
Challenges: Medication availability, overlap of activities for the same target audience, unequal cost sharing (especially with vitamin A integration), drug shortages, and unequal distribution among districts.
Azithromycin Management: Azithromycin received by national entities, centrally stored in Niamey (monitored for inventory/expiry), transported 7 days pre-distribution, with local community relays managing district distribution.
Drug Waste: Empty bottles are incinerated post-campaign; partly-used bottles are also destroyed three days after reconstitution. Wastage rate is deemed “insignificant.”
Allocation Request for 2026:
- Two extra regions (Diffa and Tilibéri) planned for distribution, targeting 659,000 inhabitants.
- Estimated 9.8 million doses needed (based on national census data and three MDA rounds).
- Planned MDA months: February, June, November.
- Implementing partners: Ministry of Health, CRISP, co-administration platform (testing integration with spatial mosquito repellent).
- Summary: 3,475,000 children, 9,832,000 doses
Supply Chain Discussion & Update
Standardization of yield at four doses per bottle was discussed. It was confirmed that four treatments per bottle is the default, but individual country requests are addressed separately. The proposed next step is to discuss the standardization of expectations and dosing at the REACH Network annual meeting.
ITI provided an overview of forecasts for 2026 azithromycin supply for child survival in four countries (Burkina Faso, Mali, Niger, Nigeria).
- Forecast assumptions: 95% allocation of expressed needs, 2% for losses/waste, 4 treatments per bottle, no remaining stock from previous distributions.
- Total forecasted needs for 2026: ~58 million treatments.
- Burkina Faso: Two campaigns (June, November), 6 regions, ~4.4 million treatments.
- Mali: Two campaigns (June, December), 8 regions, ~8.23 million treatments.
- Niger: Three campaigns (February, June, November), 7 regions, ~10 million treatments.
- Nigeria: Two campaigns per state (March-November), 11 states, ~35 million treatments (61% of total demand).
- 58 million treatments requires 14.5 million bottles of powder for oral suspension, representing a significant logistical effort.
- Logistics involve three manufacturers, coordination across four continents and seven time zones, requiring extensive communication and 15-20 shipments. Success depends on precision, detailed planning, and close collaboration.
Eligibility Criteria and APACS Recommendations
1. APACS members discussed and provisionally accepted the proposed criteria for allocation of donated azithromycin. Countries are eligible to request azithromycin for use in their child survival program if the following criteria are met:
- There is evidence that mortality levels are above an agreed threshold (to be determined)
- There is funding available to support azithromycin distribution
- There is a plan for monitoring AMR
- The administration of azithromycin for child survival is being done in the context of a broader package of child survival interventions
- The health ministry signs a memorandum of understanding with ITI through The Task Force for Global Health on appropriate storage, management, and distribution of donated azithromycin.
2. APACS members discussed and provisionally accepted the proposed recommendation categories for allocation of donated azithromycin.
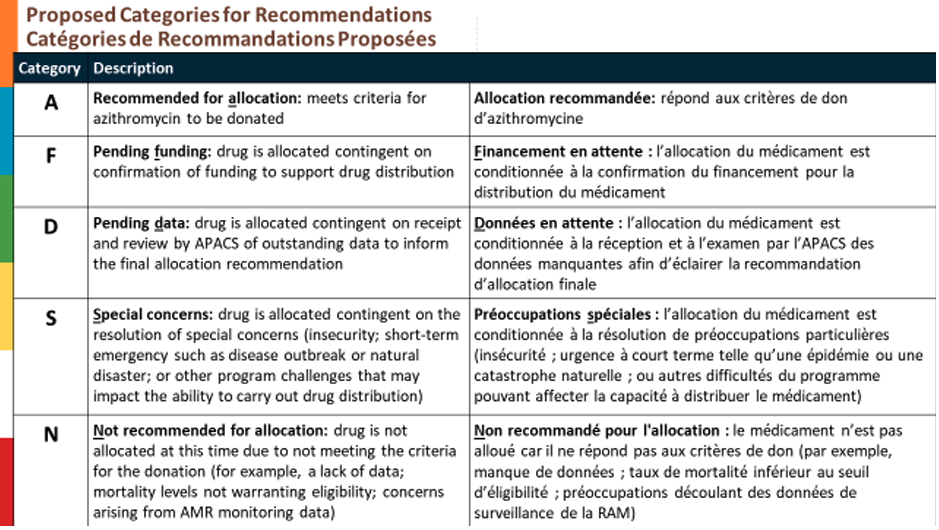
3. APACS members recommended approval of Niger’s 2026 request for donated azithromycin for mass drug administration (MDA) in all seven regions requested. In making this recommendation, APACS members discussed the following concerns:
- WHO guidelines recommend biannual MDA with azithromycin for child survival, i.e., at approximately 6-month intervals. Niger is requesting azithromycin for three rounds of MDA in 2026. APACS members noted that azithromycin production may not be adequate to supply three, rather than two, rounds. APACS did not make a recommendation regarding two versus three rounds. APACS recommends discussion with ITI to match 2026 supply to demand.
- Mortality data for Agadez, Tillaberi, and Diffa regions are based on a 2021 survey powered at national level. The under-five mortality rate point estimate for Agadez was 37 deaths per 1000 live births: below the threshold of 50 deaths per 1000 live births proposed by Niger for initiating child survival MDA, but above the proposed threshold for stopping MDA (25 deaths per 1000 live births). MDA for child survival has begun already in Agadez, and the request was therefore positioned as one for continuing MDA in a population for which the most recent child mortality estimate was above the proposed stopping threshold. This point raised several issues for further deliberation, including 1) evidence-based regional starting and stopping criteria for MDA; 2) the appropriate administrative level for which mortality surveys should be powered and recommendations made; and 3) the frequency of mortality surveys for purposes of allocating donated azithromycin (i.e., the point at which the data become ‘stale’).
- Based on the 2021 mortality data for Agadez and the need for up-to-date data to support evidence-based drug allocations, APACS recommended that the planned mortality survey planned for Agadez in 2026 be accelerated.
Because of the likelihood that additional information and deliberation will influence future recommendations, this APACS recommendation and the principles underlying it should be regarded as non-precedent-setting.
Concluding Remarks
The meeting chair thanked all participants for their patience and engagement in a rich discussion. Deep gratitude was expressed to APACS members, country liaisons, donors, the APACS steering committee, the APACS Secretariat, and observers for their participation and input.
The inaugural nature of the meeting was reiterated, acknowledging that not all details were perfectly aligned, and emphasizing the commitment to improving the deliberative process to make the best recommendations for children benefiting from donated azithromycin. Feedback on the meeting and process is welcomed via a new email address: secretariat@apacs.africa.